MedAlliance SELUTION SLR is the first DEB to receive coronary de novo IDE approval, its fourth FDA IDE DEB Approval
PR Newswire
11 Jan 2023, 06:30 GMT+10
 |
GENEVA, Jan. 11, 2023 /PRNewswire/ -- SELUTION SLR, MedAlliance's novel sirolimus-eluting balloon, has received conditional FDA Investigational Device Exemption (IDE) approval to initiate its pivotal clinical trial for the treatment of coronary de novo lesions.
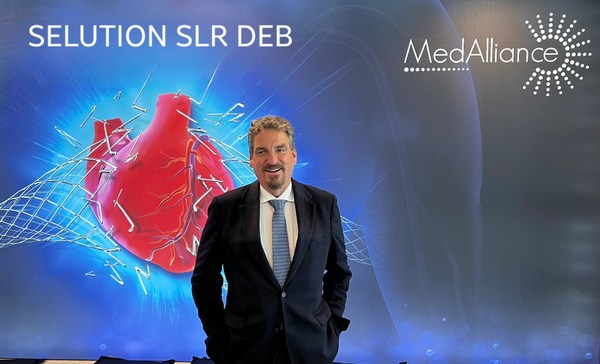
MedAlliance SELUTION SLR is the first DEB to receive coronary de novo IDE approval , its fourth FDA IDE DEB Approval
This comes less than eight months after the company received its first IDE approval for SELUTION SLR in the treatment of below-the-knee (BTK) indications; as well as occlusive disease of the superficial femoral artery (SFA); and coronary In-Stent Restenosis (ISR).
Enrollment of the SELUTION SLR coronary de novo study will begin in the US within the next few months.
This will complement the substantial experience that the company has already gained with the SELUTION DeNOVO trial in Europe (ClinicalTrials.gov Identifier: NCT04859985). More than 800 patients of the 3,326 planned have been enrolled in this ground-breaking coronary randomized controlled study comparing SELUTION SLR vs. any limus drug-eluting stent (DES). The study is powered to demonstrate superiority of SELUTION SLR drug-eluting balloon (DEB) over DES in coronary de novo artery disease. This is the largest DEB study ever initiated and has the potential to change medical practice where implants (metal stents) have been the standard of care for more than 30 years.
"Treatment of de novo coronary arteries with drug-eluting balloons is a breakthrough in revascularization of coronary artery disease. The SELUTION SLR coronary de novo study is the first of its kind in the USA and will provide important data on the efficacy and safety of sirolimus- eluting balloon as a viable alternative to drug-eluting stent, leaving nothing behind post-PCI and eliminating in-stent restenosis and related complications," said Dr. Ron Waksman, Professor of Cardiology at Georgetown University, Director of Cardiovascular Research at MedStar Heart and Vascular Institute, Washington DC and Chairman of the MedAlliance Coronary Study Steering Committee.
"Coronary de novo lesions are the largest potential opportunity for use of DEB's: the data has shown clearly that DES don't work well in small vessels, long, or bifurcated lesions or in patients with diabetes or risk of high bleeding complications. These patients represent 60% of all patients currently treated with DES, who may now benefit from this exciting new DEB technology," added Jeffrey B. Jump, Chairman and CEO of MedAlliance.
SELUTION SLR was awarded CE Mark Approval for the treatment of peripheral artery disease in February 2020 and for the treatment of coronary artery disease in May 2020.
MedAlliance's unique DEB technology involves MicroReservoirs which contain a mixture of biodegradable polymer intermixed with the anti-restenotic drug sirolimus applied as a coating on the surface of an angioplasty balloon. These MicroReservoirs provide controlled and sustained release of the drug for up to 90 days.
SELUTION SLR 014 PTCA is commercially available in Europe, Asia, the Middle East and the Americas (outside USA) and most other countries where the CE Mark is recognized. Over 10,000 coronary units have already been used for patient treatment in routine clinical practice or as part of clinical trials.
About MedAlliance
MedAlliance is a medical technology company which announced a staged acquisition by Cordis in October 2022. It is headquartered in Nyon, Switzerland, MedAlliance specializes in the development of ground-breaking technology and commercialization of advanced drug device combination products for the treatment of coronary and peripheral artery disease. For further information visit: www.medalliance.com
 Share
Share
 Tweet
Tweet
 Share
Share
 Flip
Flip
 Email
Email
Watch latest videos
Subscribe and Follow
Get a daily dose of Asia Bulletin news through our daily email, its complimentary and keeps you fully up to date with world and business news as well.
News RELEASES
Publish news of your business, community or sports group, personnel appointments, major event and more by submitting a news release to Asia Bulletin.
More InformationInternational
SectionNative leaders, activists oppose detention site on Florida wetlands
EVERGLADES, Florida: Over the weekend, a diverse coalition of environmental activists, Native American leaders, and residents gathered...
Beijing crowds cheer AI-powered robots over real soccer players
BEIJING, China: China's national soccer team may struggle to stir excitement, but its humanoid robots are drawing cheers — and not...
COVID-19 source still unknown, says WHO panel
]LONDON, U.K.: A World Health Organization (WHO) expert group investigating the origins of the COVID-19 pandemic released its final...
Fox faces $787 million lawsuit from Newsom over Trump phone call
DOVER, Delaware: California Governor Gavin Newsom has taken legal aim at Fox News, accusing the network of deliberately distorting...
DeepSeek faces app store ban in Germany over data transfer fears
FRANKFURT, Germany: Germany has become the latest country to challenge Chinese AI firm DeepSeek over its data practices, as pressure...
Canadian option offered to Harvard graduates facing US visa issues
TORONTO, Canada: Harvard University and the University of Toronto have created a backup plan to ensure Harvard graduate students continue...
Business
SectionTech stocks slide, industrials surge on Wall Street
NEW YORK, New York - Global stock indices closed with divergent performances on Tuesday, as investors weighed corporate earnings, central...
Canada-US trade talks resume after Carney rescinds tech tax
TORONTO, Canada: Canadian Prime Minister Mark Carney announced late on June 29 that trade negotiations with the U.S. have recommenced...
Lululemon accuses Costco of selling knockoff apparel
Vancouver, Canada: A high-stakes legal showdown is brewing in the world of athleisure. Lululemon, the Canadian brand known for its...
Shell rejects claim of early merger talks with BP
LONDON, U.K.: British oil giant Shell has denied reports that it is in talks to acquire rival oil company BP. The Wall Street Journal...
Wall Street extends rally, Standard and Poor's 500 hits new high
NEW YORK, New York - U.S. stock markets closed firmly in positive territory to start the week Monday, with the S&P 500 and Dow Jones...
Canadian tax on US tech giants dropped after Trump fury
WASHINGTON, D.C.: On Friday, President Donald Trump announced that he was halting trade discussions with Canada due to its decision...