First American Patient Treated with VenusP-Valve Under Compassionate Use
PR Newswire
22 Jun 2022, 15:32 GMT+10
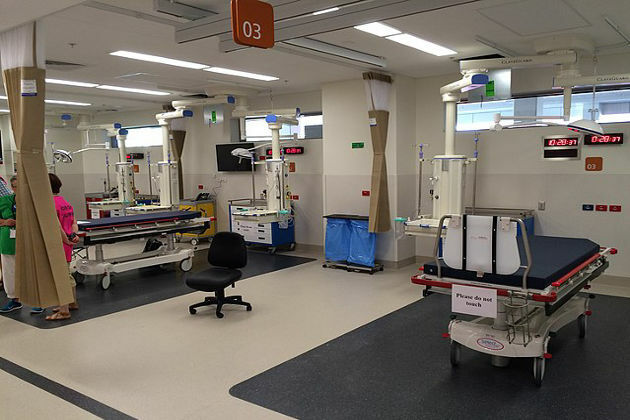
HANGZHOU, China, June 22, 2022 /PRNewswire/ -- VenusP-Valve, an in-house developed novel medical device of Venus Medtech (Hangzhou) Inc. (2500.HK, hereinafter referred to as "Venus Medtech"), recently completed its first compassionate use at University of Virginia Advanced Cardiac Valve Center in the U.S.
The procedure was performed at the Center by Professor Scott Lim's team under on-site guidance from Professor Yuan Feng and remote guidance from Professor Mao Chen, who are both from Cardiology Department, West China Hospital, Sichuan University in China. As the first-ever clinical application of a Chinese-developed heart valve product in the U.S., the case corroborates not only the unique clinical value of VenusP-Valve but also the significance of global-oriented innovation.
Although there are several transcatheter pulmonic valve replacement (TPVR) products available in the U.S. market, such as Melody TPV and Harmony TPV by Medtronic and the SAPIEN series by Edwards, they do not work for the considerable number of patients with dilated right ventricular outflow tract (RVOT). As a large-diameter product, VenusP-Valve can satisfy the clinical needs of 85% of patients in the case of large RVOT. In May 2022, VenusP-Valve was approved by the FDA for compassionate use in two patients.
This first patient was a 21-year-old man who suffered from severe pulmonary regurgitation, right ventricular dilation, pulmonary artery dilation, and left ventricular dysfunction after transannular patch (TAP) repair of Tetralogy of Fallot (TOF). The procedure was performed under local anesthesia where the patient remained conscious. Upon preoperative CT, intraoperative contrast-enhanced ultrasound, and balloon measurement, a P34-25 (valve diameter 34mm, straight length 25mm) VenusP-Valve that precisely fit the patient's anatomy was delivered through the right femoral vein. On the first postoperative day, ultrasound scan indicated a marked decrease in right ventricle volume with no pulmonary regurgitation. Left ventricular function also returned to normal. Making a sound recovery according to postoperative assessment, the patient was discharged on June 18 (within 24 hours of the procedure) local time.
"It was a great honor to work with Professor Chen and Professor Feng on the first compassionate use of VenusP-Valve in the United States", commented Professor Scott Lim after the procedure. "The product was very easy to handle as it's designed to facilitate precise positioning and delivery. The patient demonstrated significant improvement after the procedure. For the benefit of more patients, I really look forward to formal clinical trials of VenusP-Valve here as soon as possible."
Professor Chen congratulated on the first compassionate use of VenusP-Valve in the U.S. As he noted, VenusP-Valve has gained broad recognition from the global cardiology community since its first implantation in 2013. In particular, the product is a lifesaver for patients with dilated RVOT. Its transcatheter approach reduces trauma and speeds recovery, enhancing quality of life for patients in various dimensions.
Professor Feng said "VenusP-Valve is an excellent TPVR product. Its unique double flared-end design enables one-time delivery of stent and valve and simplifies the procedural workflow, making it a powerful tool for transcatheter treatment of pulmonary regurgitation. It is a pride of China to launch such a self-developed innovation in the U.S."
In April 2022, VenusP-Valve became the first Chinese-developed self-expanding TPVR product to receive CE marking under the Medical Devices Regulation (MDR). Uniquely designed with both flared ends, the product ensures the blood flow of branchial artery with bare stents at the outflow end. It provides a stable multi-point anchoring system and enables easy delivery, with no need for pre-stenting before the procedure. Available in a variety of specifications with extensive applicability, the product is able to meet the needs of 85% of patients. VenusP-Valve is also undergoing review and approval with the Chinese National Medical Products Administration (NMPA) and is expected to be marketed within 2022.
During its nine-year clinical use since 2013, VenusP-Valve has been applied to nearly 300 cases for humanitarian reasons, spanning more than 20 countries and regions in Asia, Europe, North America, and South America. In March 2021, VenusP-Valve received special use authorization from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for use in designated medical institutions.
VenusP-Valve has impressive clinical data backing up its long-term safety and efficacy. According to two-year follow-up result of the clinical study in Europe, the product demonstrated 100% procedural success, with no reoperation or death observed in two years. In addition, moderate and severe pulmonary regurgitation plunged from 16.88% and 83.12% preoperatively to 0%. The data suggest excellent performance, robust safety and reliability, and drastic and steady improvements in patients' cardiac function.
"The first compassionate use of VenusP-Valve accentuates the urgent clinical need and high regulatory recognition of the product", said Eric Zi, Founder, Executive Director, and General Manager of Venus Medtech. "VenusP-Valve is undergoing clinical trials or marketing application in many mainstream countries. It is an exemplar for Chinese-developed innovative medical devices to reach global markets. We have full confidence in the rapid global launch of the product as well as the benefits it brings to patients worldwide."
SOURCE Venus Medtech (Hangzhou) Inc.

 Share
Share
 Tweet
Tweet
 Share
Share
 Flip
Flip
 Email
Email
Watch latest videos
Subscribe and Follow
Get a daily dose of Asia Bulletin news through our daily email, its complimentary and keeps you fully up to date with world and business news as well.
News RELEASES
Publish news of your business, community or sports group, personnel appointments, major event and more by submitting a news release to Asia Bulletin.
More InformationInternational
SectionTikTok building U.S.-only app amid pressure to finalise sale
CULVER CITY, California: TikTok is preparing to roll out a separate version of its app for U.S. users, as efforts to secure a sale...
Trump defends use of 'Shylock,' citing ignorance of slur
WASHINGTON, D.C.: President Donald Trump claimed he was unaware that the term shylock is regarded as antisemitic when he used it in...
Summer travel in chaos as French air traffic controllers walk off job
PARIS, France: A strike by French air traffic controllers demanding improved working conditions caused significant disruptions during...
Congress weighs Medicaid cuts, sparking alarm in small-town hospitals
OMAHA, Nebraska: With Congress considering cuts totaling around US$1 trillion to Medicaid over the next decade, concerns are rising...
Gas station blast injures 40 in Rome, kids narrowly escape
ROME, Italy: Quick thinking by emergency responders helped prevent greater devastation after a gas station explosion in southeastern...
Weapons pause by Trump signals shift away from foreign wars
WASHINGTON, D.C.: President Donald Trump is drawing praise from his core supporters after halting key arms shipments to Ukraine, a...
Business
SectionBirkenstock steps up legal battle over fakes in India
NEW DELHI, India: Birkenstock is stepping up its efforts to protect its iconic sandals in India, as local legal representatives conducted...
Beijing hits back at EU with medical device import curbs
HONG KONG: China has fired back at the European Union in an escalating trade dispute by imposing new restrictions on medical device...
Wall Street reels after Trump invokes new tariffs
NEW YORK, New York - Monday's trading session saw mixed performances across U.S. and global markets, with several major indices posting...
Trump admin allows GE to restart engine sales to China’s COMAC
WASHINGTON, D.C.: The U.S. government has granted GE Aerospace permission to resume jet engine shipments to China's COMAC, a person...
Saudi Aramco plans asset sales to raise billions, say sources
DUBAI, U.A.E.: Saudi Aramco is exploring asset sales as part of a broader push to unlock capital, with gas-fired power plants among...
Russia among 4 systemic risk countries for Italian banks
MILAN, Italy: Italian regulators have flagged four non-EU countries—including Russia—as carrying systemic financial risk for domestic...